Tannase (EC 3.1.1.20) belongs to the class of hydrolases. It catalyzes the hydrolysis of digallate to gallate. The systematic name of this enzyme class is tannin acylhydrolase. Other names in common use include tannase S, and tannin acetylhydrolase. Tannase is a natural adaptive intracellular/extracellular inducible hydrolase and placed in the esterase superfamily. Tannase can be obtained from plants, animals and microorganisms, but the microbial-derived tannase is more extensive because its stability is higher than that of plant and animal sources.
Structure
Natural B. subtilis tannase consists of 9.3% α-helix, 33.6% parallel β-sheet, 17.2% β-turn and 39.9% random coil. The β conformation plays a dominant role in tannase activity, and the secondary structure of tannase is stringently dependent on its microenvironment (temperature and pH). B. Subtilis tannase scanning probe microscopyic analysis (SPM) showed that tannase showed different degrees of aggregation, the structure is similar to round or oval, the size is different, and the average diameter is 44 nm. The crystal structure of tannase is a small plate-like crystal. Three-dimensional structural analysis of L. plantarum tannase revealed that it exhibited α/β structure with 18 α-helices and 13 β-strands.
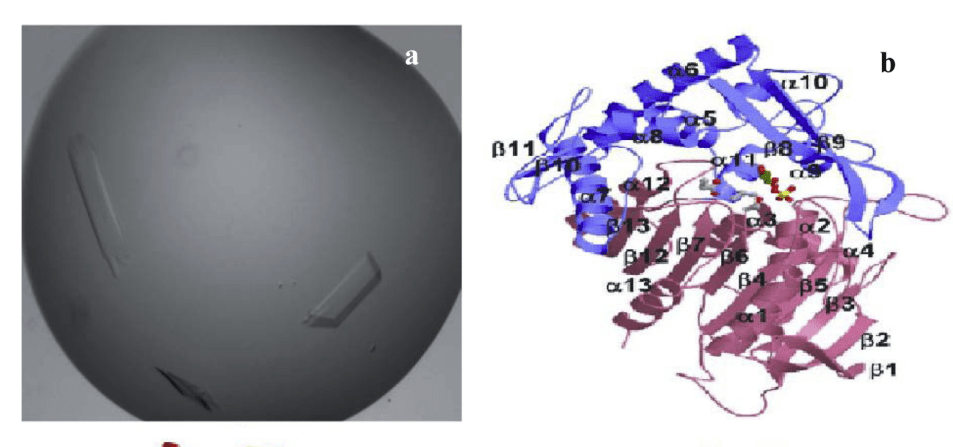 Figure 1. Crystal structure (a) and three dimensional ribbon of L. plantarum tannase (b). (Jana A. 2014)
Figure 1. Crystal structure (a) and three dimensional ribbon of L. plantarum tannase (b). (Jana A. 2014)
Physicochemical properties
The properties of tannase vary from species to species. Tannase has a molecular weight of 46.5-90 kDa and exists as a monomer, while tannase from Rhodococcus sp. and L. plantarum contains two subunits. So far, all tannases from yeast and fungi are glycoproteins, but there seems to be no such post-translational modification in bacteria. Tannase is an acidic protein with an optimum pH range of 4.5-7.0. The optimal temperature of different kinds of tannase is different, and the optimum temperature of most bacterial tannase is between 30 and 40 °C. When methyl gallate was used as a substrate and the reaction temperature was 30-40 °C, the bacterial tannase substrate affinity (Km) from Selenomonas ruminantium and Enterobacter sp. was 1.6 and 3.7, respectively. More than 28% of bacterial tannase requires metal ions as a cofactor to stimulate its maximum catalytic efficiency. It has also been found that the activity of B. subtilis tannase is increased in polar protic solvents such as glycerol, isopropanol, ethanol, methanol and isoamyl alcohol, while butanol, acetic acid and acetone reduce the activity of tannase.
Catalytic Mechanism
Tannase is the most studied enzyme in tannin biodegradation. It catalyzes the hydrolysis of esters and depside bonds of various substrates, including gallanonins, gallic acid esters, epigallocatechin gallate and epicatechin gallate, releasing gallic acid and glucose. L. plantarum tannase consists of two domains, one α/β-hydrolase domain (residues 4-204 and 396-469) and a "lid" domain (residues 205-395). The glycerol molecule in the cryoprotectant solution binds to the active site of the tannase, which mimick the binding of the galloyl moiety to its substrate. The active site of tannase is located in the α/β-hydrolase domain, of which three amino acid residues Ser163, Asp419 and His451 are catalytic triads. L. plantarum tannase contains 18 α-helices and 13 β-strands, and Ser163 is present in the Gly161-X-Ser163-X-Gly165 pentapeptide motif between β6 and α6. In the active site, Ser163 forms a hydrogen bond with the NE2 atom of the His451 imidazole ring. During the reaction, His451 deprotonated the hydroxyl group of Ser163, and Ser163 was stabilized by hydrogen bonding with Asp419.
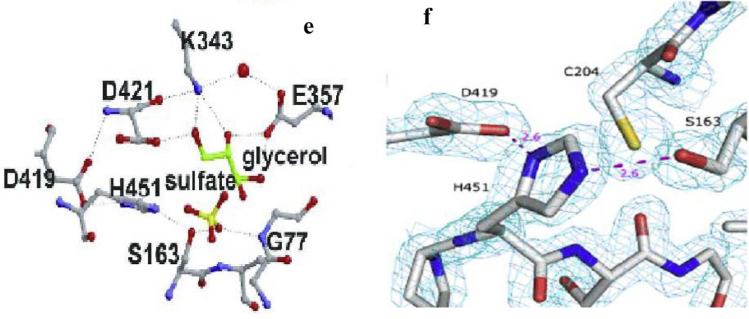 Figure 2. Active site (e) and catalytic triad region of L. plantarum tannase (f). (Jana A. 2014)
Figure 2. Active site (e) and catalytic triad region of L. plantarum tannase (f). (Jana A. 2014)
Application
Tannase participates in fruit ripening by breaking the ester bonds of glucose with chebulinic, gallic and hexahydrophenic acid. Tannase is also used in the food, feed, beverages, pharmaceutical, and chemical industries to produce gallic acid, instant tea, coffee flavored refreshing drinks and acron wines. In addition, tannase is used to clarify beer and juice, improve the flavor of the wine and make animal feed. In the chemical industry, tannase can be used to analytical probe preparation, determine the structure of naturally occurring gallic acid esters, detect cancer cells, and treat tannins-containing wastewater in the olive oil and leather industries.
References
-
Jana, A., Halder, S.K., Banerjee, A., Paul, T., Pati, B.R., Mondal, K.C., Mohapatra, P.K.D. Biosynthesis, structural architecture and biotechnological potential of bacterial tannase: A molecular advancement. Bioresource Technology, 2014, 157:327–340.
-
Rivas, B., Rodríguez, H., Anguita, J., Muñoz, R. Bacterial tannases: classification and biochemical properties. Appl Microbiol Biotechnol, 2019, 103(2):603-623.


 Figure 1. Crystal structure (a) and three dimensional ribbon of L. plantarum tannase (b). (Jana A. 2014)
Figure 1. Crystal structure (a) and three dimensional ribbon of L. plantarum tannase (b). (Jana A. 2014)
 Figure 2. Active site (e) and catalytic triad region of L. plantarum tannase (f). (Jana A. 2014)
Figure 2. Active site (e) and catalytic triad region of L. plantarum tannase (f). (Jana A. 2014)