Our Products Cannot Be Used As Medicines Directly For Personal Use.


Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Protelomerase
Related Reading
Telomeres play an important role in maintaining chromosomal stability and cell viability in cells of different species. Telomerase is an enzyme responsible for the elongation of telomeres in cells. It is a nucleoprotein reverse transcriptase that adds DNA to the ends of chromosomes to extend and repair the telomeres lost due to DNA replication and increase the number of cell divisions. Telomerase plays an important role in maintaining the integrity of the genome, cell’s long-term activity and potential proliferation.
However, in normal human cells, the activity of telomerase is tightly regulated. Only a few cells such as stem cells and germ cells can detect telomerase activity. When the cells differentiate and mature, the activity of telomerase will gradually disappear. In addition, telomerase is reactivated in tumors, which may participate in malignant transformation.
Among bacteria and viruses, including plant pathogen Agrobacterium tumefaciens, linear plasmid prophage, Escherichia coli, etc., there is an easy and common solution to the problem of chromosome ends. These organisms have linear chromosomes or replicons with covalently closed hairpin termini. Bidirectional DNA replication starts from internal origin and produces a circular dimer with inverted telomere repeats at the junction between the two copies. Then, this circular replication intermediate is broken down into two linear chromosomes with covalently closed DNA hairpins by a specialized enzyme called protelomerase.
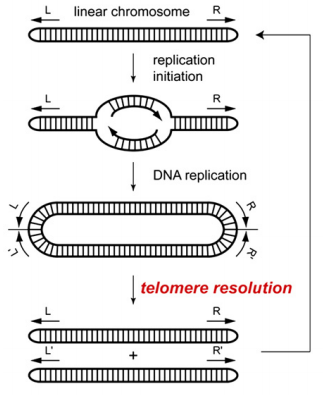 Fig 1. Protelomerase resolves replicated hairpin telomeres (Hideki, A.; et al. 2007)
Fig 1. Protelomerase resolves replicated hairpin telomeres (Hideki, A.; et al. 2007)
Structure of protelomerase
The dimer crystal structure of the protelomerase TelK from Klebsiella oxytoca phage fKO2 was reported. Each monomer of TelK is composed of an N-terminal domain and a catalytic domain, a long α-helix (helix K) as a linker to connect these two parts, forming a structure similar to tyrosine recombinase. Each TelK monomer has an oval shape (110A˚x 35A˚x 60A˚), the N-terminal domain has an insertion called muzzle, which contacts the opposite subunit enforces an offset in the path of the DNA across the dimer interface. A C-terminal DNA binding domain called stirrup exists only in TelK, it is necessary to resolve hairpin telomeres. The dimers formed by TelK provide a large contact surface for DNA, this surface maintain the 44 bp DNA substrate in a curved configuration with a central discontinuity in the DNA helical axis. The contact area between the two subunits is large, and the interaction between the subunits mainly occurs between the N-terminal domains and between the catalytic domains. When it’s working, the DNA is completely encapsulated by the protein, which provides a relatively fixed environment during the catalysis process to keep the double-stranded DNA in the strained conformation.
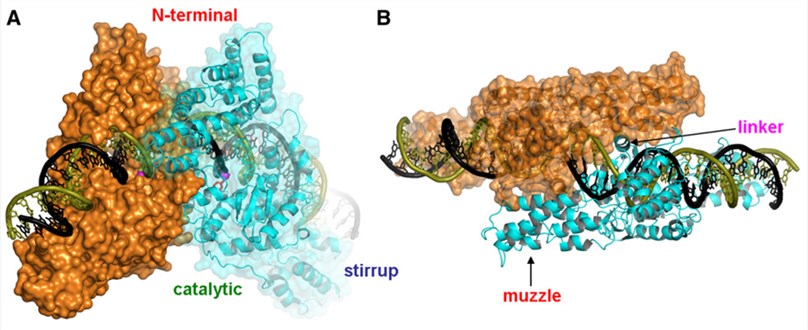 Fig 2. A. A view perpendicular to the two-fold noncrystallographic symmetry axis that relates two TelK subunits colored in orange and cyan B. Top view along the two-fold axis (Hideki, A.; et al. 2007)
Fig 2. A. A view perpendicular to the two-fold noncrystallographic symmetry axis that relates two TelK subunits colored in orange and cyan B. Top view along the two-fold axis (Hideki, A.; et al. 2007)
Application
Some protelomerases have been identified as not having the effect of solving telomere problems in vivo, but have, for example, single strand annealing and ATP-dependent helicase activities. Therefore, a detailed study of this important class of enzymes will help clarify the importance of single strand annealing and DNA unwinding activities in the process of closed linear chromosome replication.
Protelomerase is not only indispensable in organisms, but also has unique significance to the biotechnology industry. There are already DNA constructs produced by protelomerase on the market as an improved cloning vector with highly repetitive sequences, which has a more stable linear structure and is less susceptible to genetic loss during replication. Protelomerase has also been used for expression in engineered E. coli cells to produce linear eukaryotic vectors that do not contain bacterial sequences. In addition, protelomerase is an important part of the amplification platform constructed by a company. The platform uses a cell-free process to produce large amounts of DNA for therapeutic and industrial applications. As antibiotic resistance genes are eliminated, safety has been improved. Linear and minimal vectors may be used as DNA vaccines or non-viral gene therapy vectors. At present, both methods have received a lot of attention and have also received a lot of investment in the biotechnology market.
References
- Hideki, A.; et al. An Interlocked dimer of the protelomerase TelK distorts DNA structure for the formation of hairpin telomeres. Molecular Cell. 2007, 27(6): 901-913.
- Nikolai, V.R. Mechanisms of replication and telomere resolution of the linear plasmid prophage N15. FEMS Microbiol Letter. 2003, 221(1): 1-6.