Our Products Cannot Be Used As Medicines Directly For Personal Use.


Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
PNGase
Related Reading
N-glycosylation is one of the most common post-translational modifications in proteins and is conserved in all life forms (eukaryotes, bacteria and archaea). N-glycosylation in eukaryotes is essential for various biological events, including signal transduction, protein regulation, cell-cell interactions, and tumor metastasis, etc. Peptide: N-glycanase (PNGase) is an important part of the endoplasmic reticulum-related protein degradation pathway, which de-glycosylates misfolded glycoproteins, thereby promoting the degradation of their proteasome. PNGase belongs to the transglutaminase superfamily and contains a catalytic triad of Cysteine, Histidine and Aspartate, which is necessary for its enzyme activity.
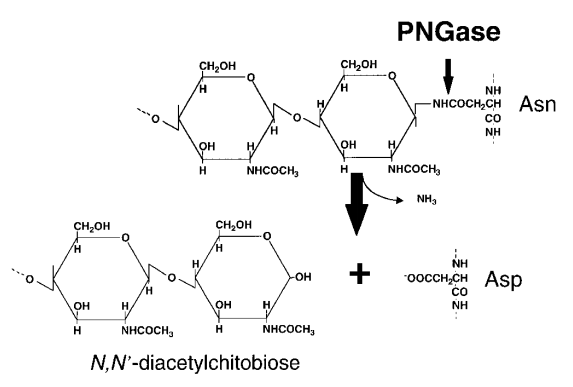 Fig1. Schematic representation of enzymatic reaction of peptide: N-glycanase (PNGase) acting on an asparagine-linked glycoprotein (Suzuki, T. 2002)
Fig1. Schematic representation of enzymatic reaction of peptide: N-glycanase (PNGase) acting on an asparagine-linked glycoprotein (Suzuki, T. 2002)
Identification of the gene coding PNGase
In 2000, the gene encoding PNGase was identified in S. cerevisiae by isolating mutants defective in PNGase activity and then identifying the causative gene locus. After detailed analyses of multiple strains, a single strain (no.352) was identified as a mutant lacking PNGase activity due to a single locus mutation, and the mutation that caused PNGase activity defect was designated as png1-1. Then through various analyses, the png1-1 locus was located on the left arm of chromosome XVI. Finally, by enzyme assays of deletion mutants of the left arm of chromosome XVI, the causative ORF, YPL096w was identified. Soon after the PNG1 allele was identified, the expression of the protein in E. coli confirmed the activity of PNGase. The gene encodes this protein containing 363 amino acids (42.5 kDa), mainly located in the cytoplasm and nucleus.
Substrate recognition mechanism of PNGase
High-throughput chemical screening of more than 100,000 compounds revealed that carbobenzyloxy-Val-Ala-Asp-fluoromethyl ketone (Z-VAD-fmk) is an irreversible PNGase inhibitor, this inhibition may be caused by covalent modification of the cysteine in the active site of the enzyme. On the other hand, PNGase is inhibited by haloacetoamidyl derivatives of carbohydrates containing GlcNAc2, and this result shows the possibility of a GlcNAc2 (chitobiose) binding pocket on PNGase. In addition, the molecular model of chitobiose binding to PNGase shows that the binding site of chitosan is adjacent to but different from that of Z-VAD-fmk. There are also reports that fluorescence-labeled chloroacetamidyl chitobiose derivative (GlcNAc2-ClAc) is an outstanding inhibitor of this enzyme in vivo and in vitro.
Structure of PNGase
PNGase molecule is folded into two domains, each domain has an eight-strand antiparallel β jelly roll structure similar to many viral capsids. The two closely related all-P domains consist of an amino-terminal domain containing residues 1-135 and a carboxy-terminal domain containing residues 142-314. A short extended piece of polypeptide, residues 136-141, are the linker of the two domains at the bottom of the molecule. Domain 2 has the same topological structure as domain 1, except that the carboxy-terminal residues 308-314 form a short ninth P-strand antiparallel to strand C.
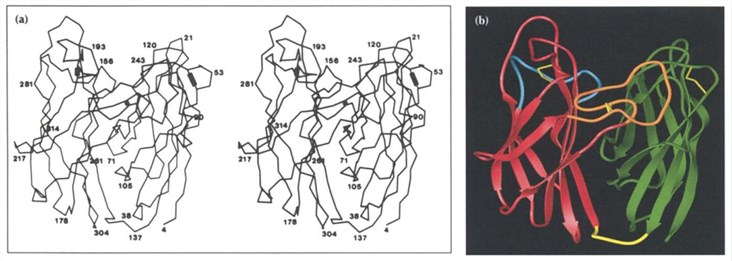 Fig 2. Structure of PNGase (Gillian E.N.; et al. 1994)
Fig 2. Structure of PNGase (Gillian E.N.; et al. 1994)
Application of PNGase
Lysosomal storage diseases and various diseases involving the nervous system are caused by the lack of endoglycosidase. N-linked glycans play an important role in all life activities. They not only constitute the structural components of the cell wall and extracellular matrix, change the various properties of proteins, but also mediate cell signal transduction (including cell-cell interactions and cell-matrix interactions). N- glycosylation can be seen on various substances such as antibodies, various proteins, and cell surfaces. Alterations in glycosylation can also be used to predict and characterize cancer and inflammatory conditions.
For this reason, PNGase can be used to characterize glycoproteins and study oligosaccharides. PNGase lacks selectivity for carbohydrate structure, leading to its wide specificity, which makes PNGase a powerful tool for studying the structure and function of glycoproteins and carbohydrates. Under normal circumstances, after the protein is processed with PNGase, gel electrophoresis, mass spectrometry and other methods can be used to characterize oligosaccharides and proteins or peptides.
References
- Gillian E.N.; et al. The three-dimensional structure of PNGase F, a glycosyl asparaginase from Flavobacterium meningosepticum. Structure. 1994, 2(11): 1049-1059.
- Hirayama, H.; et al. Physiological and molecular functions of the cytosolic peptide: N-glycanase. Seminars in Cell & Developmental Biology. 2015, 41: 110-120.
- Suzuki, T. Cytoplasmic peptide: N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. The FASEB Journal. 2002, 16(7): 635-641.