Laccase is a polyphenol oxidase belonging to the blue oxidase family and is one of the important lignocellulolytic enzymes. Laccase was first discovered in the toxicodendron vernicifluum. Subsequently, it was found that certain fungi also secreted the laccase. Laccase can catalyze the degradation of a variety of aromatic compounds, especially phenols, and is a natural environmentally friendly enzyme. The rational development of the degradation of wood fiber and some polymer compounds by laccase can reduce the use of chemicals, reduce production costs and protect the environment.
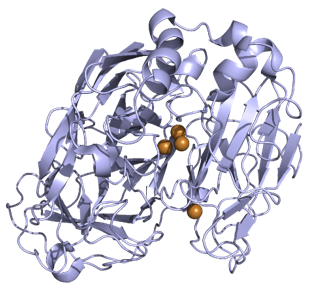 Figure 1. X-ray-determined crystal structure of laccase III. (C.F. Blanford. 2007)
Figure 1. X-ray-determined crystal structure of laccase III. (C.F. Blanford. 2007)
Sources
Laccase was first discovered by Yoshida in the oxicodendron vernicifluum in 1883. It was later discovered that certain fungi can also secrete this enzyme. These proteins have different molecular weights depending on the source, about 59-390 kD. The laccase produced by some strains is composed of several isozymes, both of which are monomeric enzymes. The enzyme molecules generally contain 4 copper atoms. The suitable reaction temperature of laccase is low, the catalytic efficiency in the acidic environment is relatively high, and the substrate specificity is obtained. The main fungi secreting laccase include Phanerochaete chrysosporium, Coriolus versicolor, Trametes versicolor, Pleurotus ostreatus, and shiitake, all of which belong to the Basidiomycetes. Laccase plays a major role in the synthesis of lignin in plants, while the laccase secreted by fungi acts exactly the opposite, mainly to degrade lignin. Therefore, the degradation of lignin by fungal laccase makes it has a wide range of applications.
Molecular Structure
The primary structure of laccase consists of Greek key beta barrel topology which constituents of approximately 500 amino acid residues in three consecutive domains. These amino acids are distributed in three domains: first domain with 150 initial amino acids, second domain with 150 and 300 amino acid, and third domain with 300–500 amino acids. The stabilization of laccase structure is due to the presence of disulfide bonds between domains I and II and between domains I and III.
Laccase is known to exist in four different Cu catalytic forms per protein unit. These four Cu ions are divided into three types of structures:
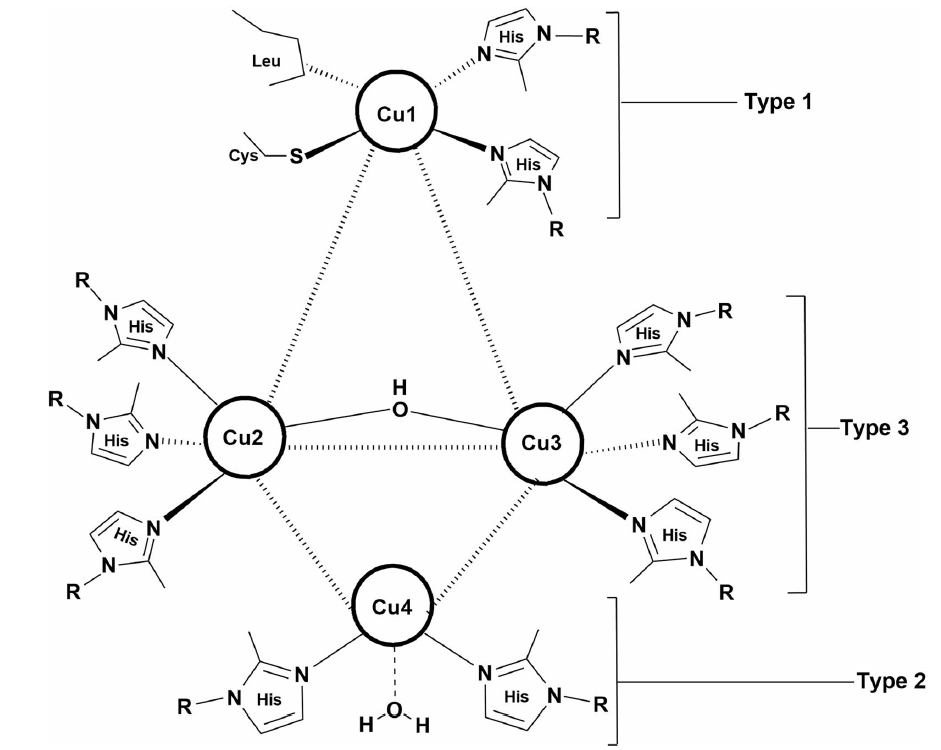 Figure 2. Copper centers of laccase: Type 1, Type 2, and Type 3. (Agrawal K. et al. 2018)
Figure 2. Copper centers of laccase: Type 1, Type 2, and Type 3. (Agrawal K. et al. 2018)
Type 1 copper confers blue color to multi-copper proteins, which is due to the intense electronic absorption caused by the covalent copper–cysteine bond. Type 1 copper has a trigonal coordination, with two histine and a cysteine as conserved equatorial ligands and one position usually variable, and in case of fungal laccases, the axial ligand is leucine or phenylalanine.
Type 2 or normal Cu site is characterized by the lack of strong absorption features in the visible region and reveals usual electron paramagnetic resonance (EPR) spectra. Type 2 copper is coordinated by two histidines residues and is strategically positioned close to Type 3 copper.
Type 3 is a binuclear center regulated by six histidines and spectroscopically characterized by an electron adsorption at 330 nm (oxidized form) and absence of an EPR signal due to the strong anti-ferromagnetical coupling between the two Type 3 copper atoms which is related to the presence of a hydroxyl bridge.
Detection Methods
At present, the most common method to detect laccase is an intuitive method for producing color change in a short reaction time by using laccase and a specific substance, such as titration method and color changing circle reaction method used with a PDA medium. For example, laccase can react with substances such as syringaldazine, guaiacol, α-naphthol, pyrogallic acid, and citric acid, and rapidly produce substances of a specific color. According to the specific color change reaction of laccase and these substances, it is determined whether the strain produces laccase. The laccase production can be estimated based on the color depth and the color circle size generated in the same time.
Fermentation Conditions
Kirk et al. obtained the best laccase production conditions in the fungi fermentation process through extensive research. The basic medium identified in this study has been widely used by researchers. The study found that the type of nitrogen source in the laccase fermentation process cannot significantly affect the laccase production, and the nitrogen dosage is one of the key factors affecting the laccase production. Nitrogen-limited culture can significantly increase the laccase production. Laccase is a copper-containing polyphenol oxidase, so Cu2+ is essential for the synthesis and activity of laccase. Low molecular aromatic compounds similar in structure to lignin or lignin-degraded fragments can also induce and significantly increase laccase production, such as guaiacol, benzyl alcohol, resveratrol, vanillic acid, Tween and so on. Limiting the carbon content can also significantly increase the production of laccase. The French Galliano et al. compared the solid and liquid medium. The lignin degradation in the solid medium was significantly higher than that in the liquid medium by the 14C radiolabeling method, and the amount of laccase produced was 15 times higher.
Application
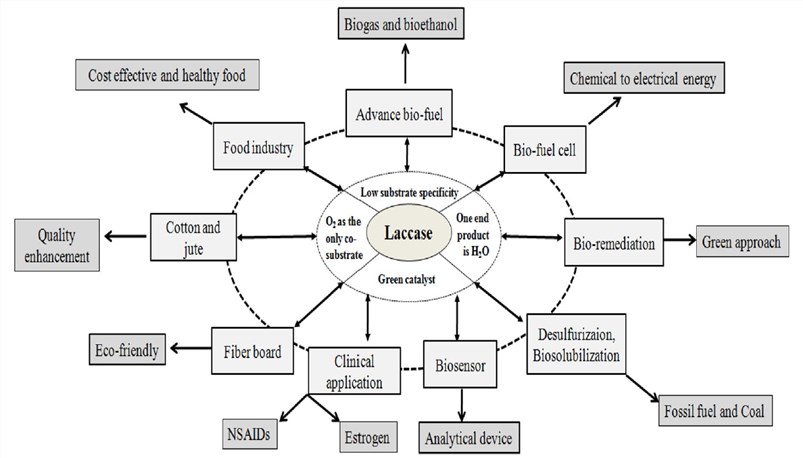 Figure 3. Applications of laccase in various industrial and biotechnological sectors. (Agrawal K. et al. 2018)
Figure 3. Applications of laccase in various industrial and biotechnological sectors. (Agrawal K. et al. 2018)
The pulping process separates the plant cellulose from the lignin, which necessitates the removal of lignin from the pulp. Biopulping mainly relies on various enzymes produced by microbial fermentation to remove lignin, thereby reducing the use of chemical agents, environmental protection, and production costs. Laccase can selectively degrade lignin and eliminate the drawbacks of mechanical pulping process, so that the production can be carried out under mild conditions of normal temperature and normal pressure, and can save equipment and energy consumption, shorten pulp production cycle and reduce production cost.
Green plants account for more than 95% of the terrestrial biomass. Rational use of green plants is an effective way to solve energy problems. The ratio of lignin, cellulose and hemicellulose in plants to plant dry weight is 20%, 45% and 20%, respectively. Among them, the degradation and utilization of lignin is the most difficult. The first problem that must be solved to completely degrade and utilize these biomass resources is the degradation of lignin. Using laccase to decompose lignin into small molecules for bio-fermentation, resulting in new energy sources such as bio-alcohol.
Laccase has also been widely used in the food industry. Increasing the stability of glucose is a major application of laccase in the food industry. The addition of laccase can improve the stability of the wine, thus reducing the amount of sulfur dioxide used. In addition, laccase can also play a very good role in fruit juice production, because the excessive oxidation of phenols will affect the quality of the product, and laccase can remove phenolic substances such as catechol in juice. Therefore, the fruit juice can be stably clarified without additional clarifying agent.
References
-
C.F. Blanford. Laccase. The Armstrong Research Group Inorganic Chemistry Laboratory. 2007. http://armstrong.chem.ox.ac.uk/laccase.html.
-
Agrawal K, Chaturvedi V, Verma P. Fungal laccase discovered but yet undiscovered. Bioresources & Bioprocessing, 2018, 5(1):4.


 Figure 1. X-ray-determined crystal structure of laccase III. (C.F. Blanford. 2007)
Figure 1. X-ray-determined crystal structure of laccase III. (C.F. Blanford. 2007)
 Figure 2. Copper centers of laccase: Type 1, Type 2, and Type 3. (Agrawal K. et al. 2018)
Figure 2. Copper centers of laccase: Type 1, Type 2, and Type 3. (Agrawal K. et al. 2018)
 Figure 3. Applications of laccase in various industrial and biotechnological sectors. (Agrawal K. et al. 2018)
Figure 3. Applications of laccase in various industrial and biotechnological sectors. (Agrawal K. et al. 2018)