Our Products Cannot Be Used As Medicines Directly For Personal Use.


Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Enzyme Purification
A variety of enzyme purification services are available at Creative Enzymes. We provide purification and quality analysis in small trial scales or large industrial scales from natural resources or production mixtures, for clinical, therapeutic, research, and chemical industries. Our services also cover the preliminary steps, as well as post-purification recovery and analysis.
Although initial characterizations of an enzyme in a mixture or sample matrix is practical, such as activity measurement and preliminary quantification, more fundamental knowledge relies on more advanced studies of the enzyme, which can only performed with pure enzyme samples. Pure enzymes also mean easier assays with less interferences. Some analysis methods, such as crystallography, are sensitive to sample purity and give desired results only with the highest samples purity. In large scale production for industrial applications, enzyme purification is directly related to product quality, in addition to regulatory requirements. Therefore, enzyme purification must be thoroughly considered and cautiously operated for both research and production purposes. However, the task is not straightforward. Many factors could change the efficiency, the yield, and stability of activity during purification, and the effects of these factors vary largely from one enzyme to another. At Creative Enzymes, we depend on the knowledgeable scientists and their years of experiences to design and perform the most suitable purification process for each enzyme. We understand that the customers may have different preferences on the purity, yield, and stability in different cases, and we will further customize the purification process to satisfy these needs.
Almost all samples need to be prepared before the actual purification. For the enzymes from cell sources, they need to be fractionated into components before purification. The first step usually involves homogenization of cells, which disrupt the cell wall to release the enzyme into the homogenate, along with other components. Depending on the cell type, the homogenization could be easy as in the case of mammalian tissue without rigid cell wall, or it may need harsher conditions such as abrasion, freezing, and high pressure due to the rigid cell wall of the plant tissue. Sometimes, additional hydrolytic enzymes or detergents are added for better extraction. The mixture is then fractionated by centrifugation, yielding a dense pellet of heavy material at the bottom of the centrifuge tube and a lighter supernatant above (Figure 1). The supernatant is again centrifuged at a greater force to yield yet another pellet and supernatant. The procedure, called differential centrifugation, yields several fractions of decreasing density, each still containing hundreds of different proteins, which are subsequently assayed for the activity being purified. Usually, one fraction will be enriched for such activity, and it then serves as the source of material to which more discriminating purification techniques are applied. The choice of temperature, pH, buffering salt, buffer strength, ionic strength, osmolarity, additives (EDTA, SDS, non-ionic detergents etc.), and homogenization technique are important of the success of purification.
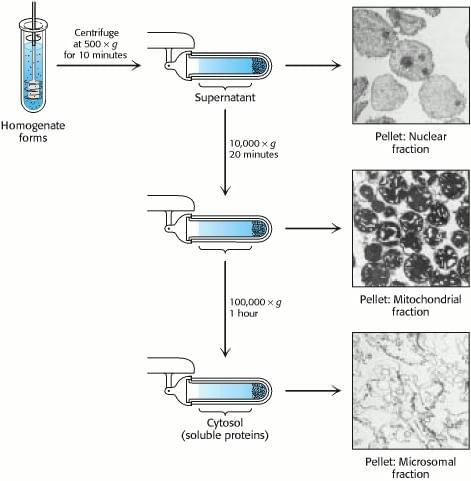 Figure 1. Differential Centrifugation.
Figure 1. Differential Centrifugation.
Reference: Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 4.1
Purification and separation of enzymes are generally based on solubility, size, polarity, and binding affinity. The production scale, timeline, and properties of the enzymes should all be considered when choosing the proper separation method.
- Solubility based separation
The principle of the type of separation is that enzyme solubility changes drastically when the pH, ionic strength, or dielectric constant changes. For example, most proteins are less soluble at high salt concentrations, an effect called salting out. The salt concentration at which a protein precipitates differs from one protein to another. Hence, salting out can be used to fractionate proteins. Salting out is also useful for concentrating dilute solutions of proteins, including active fractions obtained from other purification steps. Addition of water-miscible organic solvents such as ethanol or acetone will change the dielectric constant of the solvent and therefore precipitate the desired enzyme. Neutral water-soluble polymers can also be used for the same purpose instead of organic solvents. However, the risks of losing enzyme activity during precipitation and further separation of the added salt or polymer need to be considered.
- Size or mass based method
Because enzymes are relatively large molecules, separation based on the size or mass of molecules favors purification of enzymes, especially the ones with high molecular weight. Dialysis is a commonly used method, where semipermeable membranes are used to remove salts, small organic molecules, and peptides (Figure 2). The process usually needs a large volume of dialysate, the fluid outside the dialysis dag, and a period of hours or days to reach the equilibrium. Countercurrent dialysis cartages can also be used, in which the solution to be dialyzed flow in one direction, and the dialysate in the opposite direction outside of the membrane. Similarly, ultrafiltration membranes, which are made from cellulose acetate or other porous materials, can be used to purify and concentrate an enzyme larger than certain molecular weight. The molecular weight is called the molecular weight cutoff and is available in a large range from different membranes. The ultrafiltration process is usually carried out in a cartridge loaded with the enzyme to be purified. Centrifugal force or vacuum is applied to accelerate the process. Both dialysis and ultrafiltration are quick but somewhat vague on distinguishing the molecular weight, whereas size exclusion chromatography gives fine fractionation from the raw mixture, allowing separation of the desired enzyme from not only small molecules but also other enzymes and proteins. Size exclusion chromatography, also known as gel-filtration chromatography, relies on polymer beads with defined pore sizes that let particles smaller than a certain size into the bead, thus retarding their egress from a column. In general, the smaller the molecule, the slower it comes out of the column. Size exclusion resins are relatively “stiff” and can be used in high pressure columns at higher flow rates, which shortens the separation time. Other factors including the pore size, protein shape, column volumes, and ionic strength of the eluent could also change the result of purification.
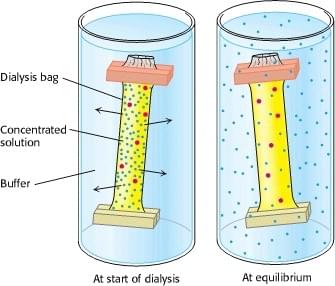 Figure 2. The scheme of dialysis. Enzyme molecules (red dots) are retained in the dialysis bag and separated from other smaller molecules (blue dots).
Figure 2. The scheme of dialysis. Enzyme molecules (red dots) are retained in the dialysis bag and separated from other smaller molecules (blue dots).
Reference: Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 4.1
- Polarity based separation
Like other proteins, enzymes can be separated on the basis of polarity, more specifically, their net charge, charge density, and hydrophobic interactions. In ion-exchange chromatography, a column of beads containing negatively or positively charged functional groups are used to separate enzymes. The cationic enzymes can be separated on anionic columns, and anionic enzymes on cationic column.
Electrophoresis is a procedure that uses an electrical field to cause permeation of ions through a solid or semi-solid matrix or surface resulting in separations on constituents on the basis of charge density. The most commonly used methods with a SDS-PAGE matrix are quite well standardized and do not differ much between labs. The distance a protein migrates in SDS-PAGE is inversely proportional to the log of its molecular radius, which is roughly proportional to molecular weight. Similarly, a matrix with gradient pH can be used in isoelectric focusing separation. A protein moves under the influence of an electrical field and stops upon reaching the pH which is the pI for the protein (net charge = 0). The matrix used can be liquid or a gel poured into either a cylindrical shape, or a flat plate.
Hydrophobic interaction chromatography (HIC) employs hydrophobic interactions to distinguish different enzymes, which are adsorbed on matrices such as octyl- or phenyl-Sepharose. A gradient of decreasing ionic strength, or possibly increasing non-polar solvent concentration can be used to elute the proteins, giving fractions that usually contain relatively high-pure enzymes. High-pressure liquid chromatography (HPLC) uses the same principle of separation of HIC, which is filled with more finely divided and tuned materials and thus allows more choices of eluents and results in better separation. Note that HPLC could be based on polarity, affinity, or both.
- Affinity or ligand based purification
Affinity chromatography is another powerful and generally applicable means of purifying enzymes. This technique takes advantage of the high affinity of many enzymes for specific chemical groups. In general, affinity chromatography can be effectively used to isolate a protein that recognizes a certain group by (1) covalently attaching this group or a derivative of it to a column, (2) adding a mixture of proteins to this column, which is then washed with buffer to remove unbound proteins, and (3) eluting the desired protein by adding a high concentration of a soluble form of the affinity group or altering the conditions to decrease binding affinity. Affinity chromatography is most effective when the interaction of the enzyme and the molecule that is used as the bait is highly specific. A special example of ligand-affinity chromatography is the Ni-NTA (nickel – nitrolotriacetic acid-agaraose) affinity chromatography. This ligand binds tightly to a 6 amino acid peptide consisting only of histidines (His6). The cDNA sequence for His6 can be appended to the cDNA coding for a given recombinant protein, thus yielding a recombinant protein which contains a His-TAG. This allows the affinity-purification of such a protein using Ni-NTA without having to design a special ligand-affinity column. Other forms of affinity chromatography include dye-ligand chromatography, immunoadsorption chromatography, and covalent chromatography.
After purification, the enzymes need to be concentrated, and sometimes lyophilized to give the pure, stable form distributed as the product or added into the final formulation. The following analysis and quality certification is necessary to confirm the enzyme is the desired one, with reasonable concentration, stability, and activity. The enrichment and certification requires experienced researchers to maintain the enzyme quality and choose the correct characterization method.
Creative Enzymes has been serving the enzyme industry for years to satisfy the needs of enzyme purification from various customers. Our services are distinguished with the high purity, rapid turnovers, and professional technical support. Please contact us for questions and inquiries for our top-rated services:
- Enzyme purification
- Affinity column for tagged enzymes (GST, Ni-NTA, protein A/G/L resins, etc.)
- Immunoprecipitation (IP)
- Ion exchange (IEC)
- Size exclusion (SEC) and gel filtration (GF)
- Hydrophobic interaction chromatography (HIC)
- Electrophoresis
- Solubility based purification
- Solubilizing inclusion body
- Enzyme recovery and refolding
- Endotoxin removal
- Quality control