
Enzymes are proteins. The “central dogma” of biochemistry emphasizes the key role of enzyme catalysis and highlights a compelling problem in biochemical evolution.
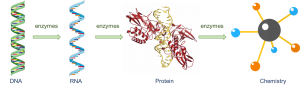
Figure 1. The “central dogma” of biochemistry.
The enzyme protein consists of one or more polypeptide chains (Fig. 2), each strand being folded into a flexible, more or less unique active conformation. The preferred three-dimensional structure is determined by a complex array of physicochemical interactions between side chains, main-chain amide groups and, in particular, the solvent water.
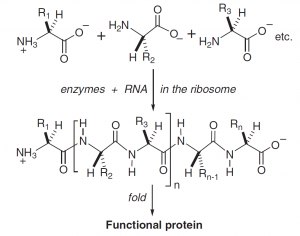
Figure 2. Protein structure and biosynthesis. (Bols M. 2010)
Functional Groups Available to Enzymes
What makes one protein different from another is the amino acid sequence. The chemical reactions involved in enzymatic catalysis are achieved by functional groups available on the side chains of the amino acids (the backbone amide groups are usually not directly involved). Nine of the 20 naturally occurring amino acids carry one of six different reactive groups (7 if the phenol and alcohol OH groups are different). These are listed in Table 1, which also gives approximations of pKas for side chain groups. The pKa value tells us how much of each ion form in a group is free of amino acids in the solution at any given pH. Although it can only indicate what may be happening in a controlled environment at the active site, pKas may be disturbed without full solvation, sometimes as many as 4-5 units. (Qualitatively speaking, this perturbation is generally beneficial to neutral species because solvation by hydrogen bonding can stabilize the ionized form more strongly.)
Table 1. Amino-acids with side-chains ionizing in the pH region. (Bols M. 2010)
| Amino acid | Side-chain functional group | Catalytic function of the group |
| Aspartic acid | 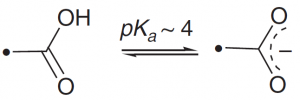 |
The COOH group can be used as a general acid, but is present near pH 7 only in a specially controlled environment. |
| Glutamic acid | 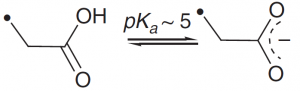 |
The carboxylate anion can be used as a general base or a nucleophile. |
| Histidine | 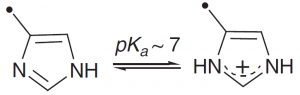 |
The imidazole group is about 50% protonated at pH 7, and is therefore the most versatile side chain group: making available general acid, general base or nucleophilic groups near pH7. |
| Cysteine | A thiolate group is a powerful nucleophile that may be present in excess of 1% in a controlled active site environment if its pKa is reduced. | |
| Lysine | NH2 is a good nucleophile, especially for C=O, its effective pKa can be reduced in a controlled active site environment. | |
| Tyrosine | 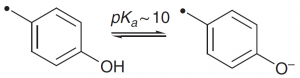 |
An oxyanion is a good nucleophile, especially for phosphorus. |
| Arginine | 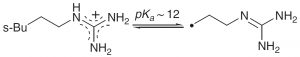 |
The guanidinium group usually remains protonated. It forms a strong, stable double H bond interaction with the CO2¯ and PO2¯ groups. |
| Serine, Threonine | 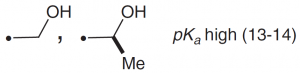 |
The OH group is not significantly ionized in the pH region, but can act as a nucleophile because the protons are removed by general bases. |
According to the standards of today’s organic or inorganic chemists, the functional groups listed in Table 1 are by no means an impressive choice of reagents. Nor can they be, because they must be operated in water and have long-term stability: any strong base, acid or electrophile will be neutralized immediately by the solvent.
Reference:
Bols M. From Enzyme Models to Model Enzymes. By Anthony J. Kirby and Florian Hollfelder [J]. Chembiochem, 2010, 11(4):581-582.
Related Products at Creative Enzymes:
Enzymes for Chemical Processing
Enzymes for Household and Daily Use
Related Services at Creative Enzymes:

Leave a Reply